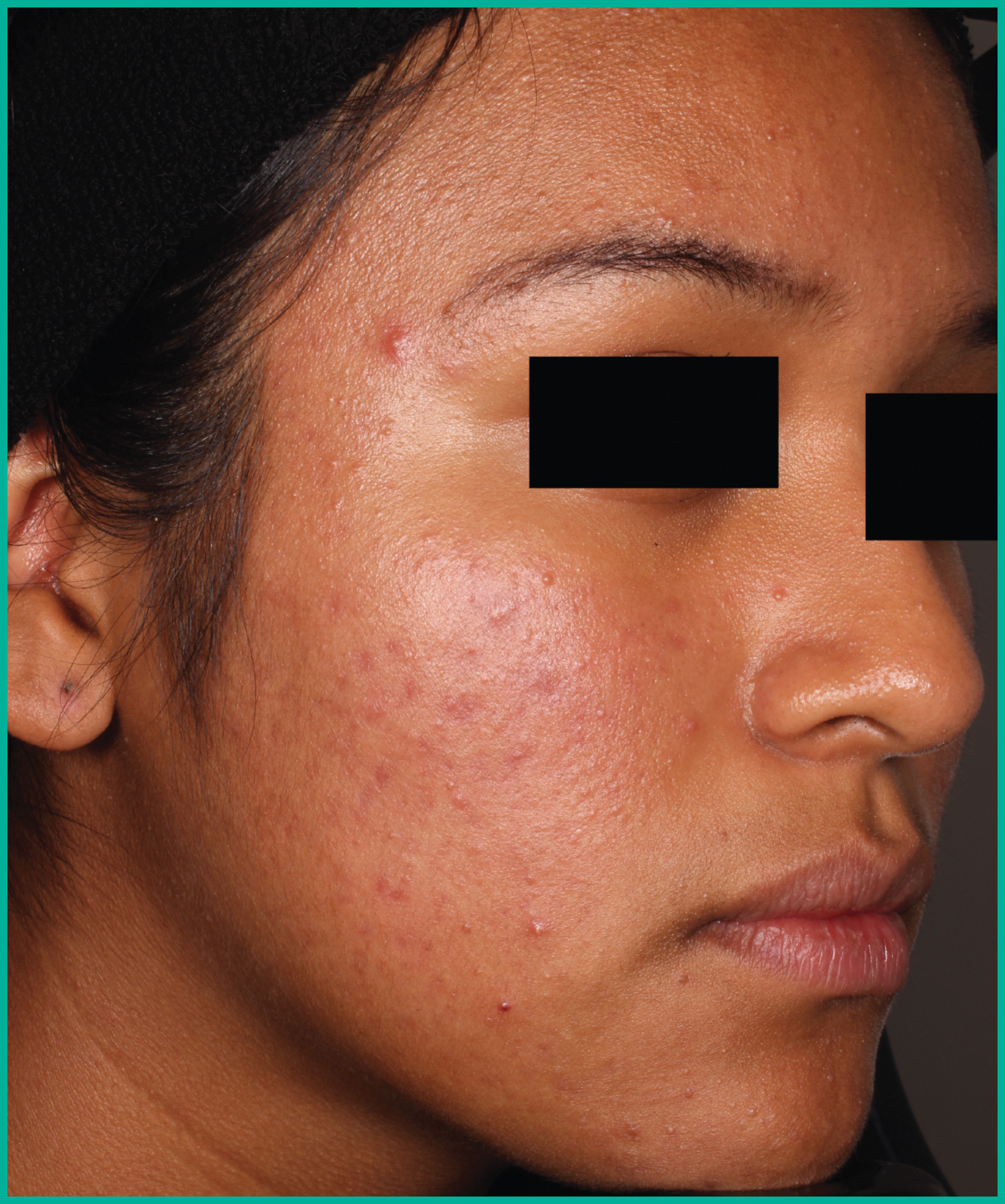
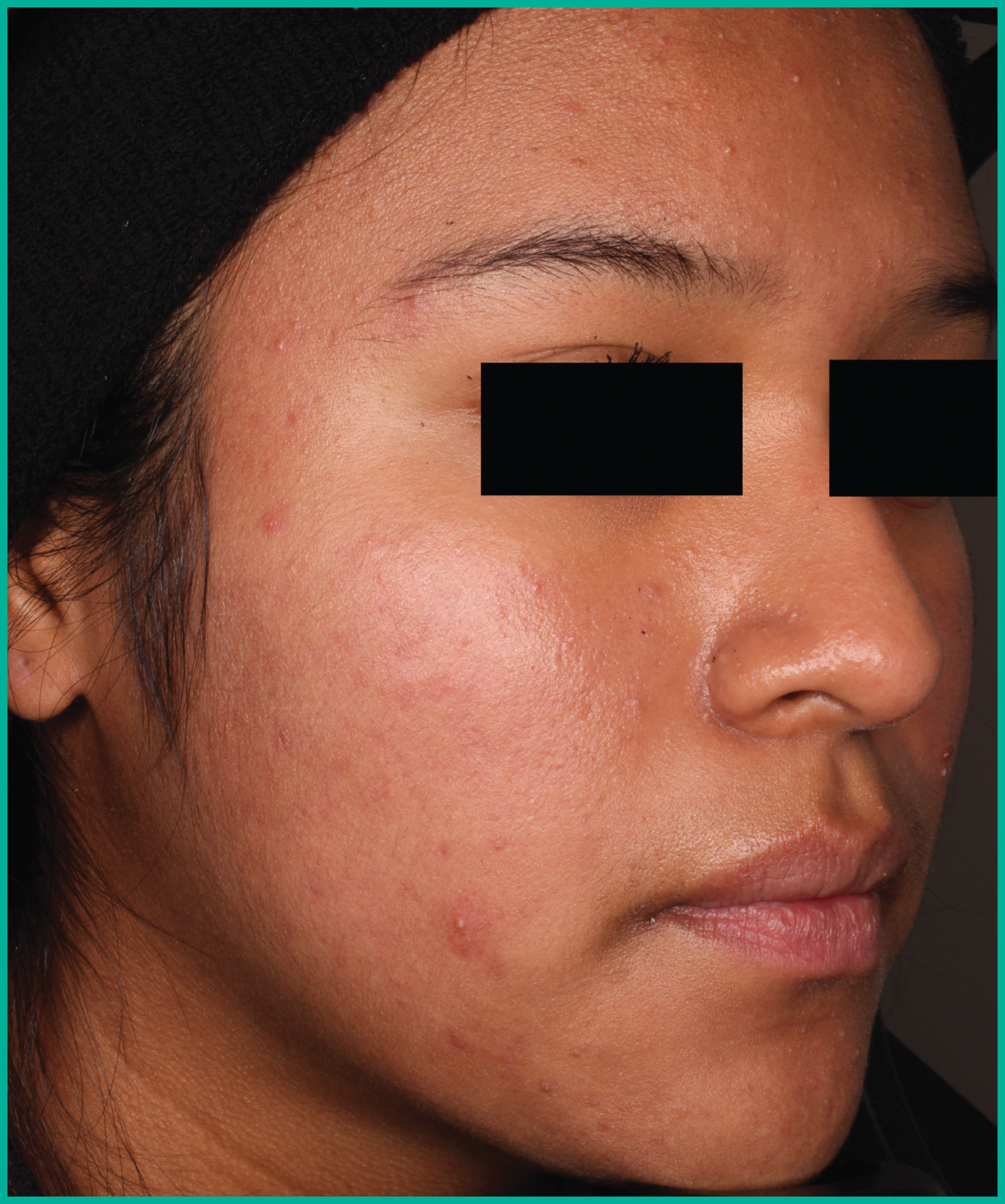
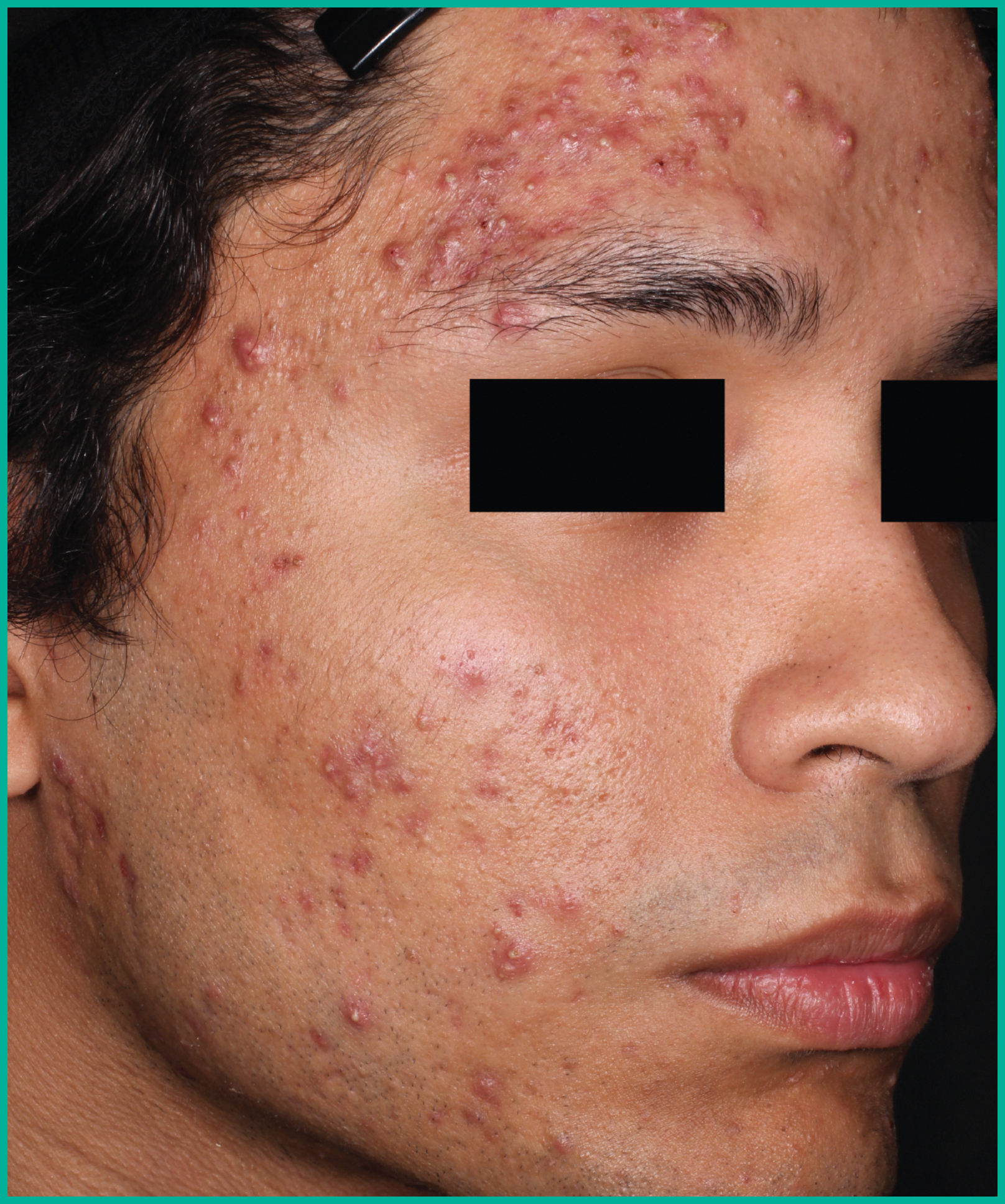
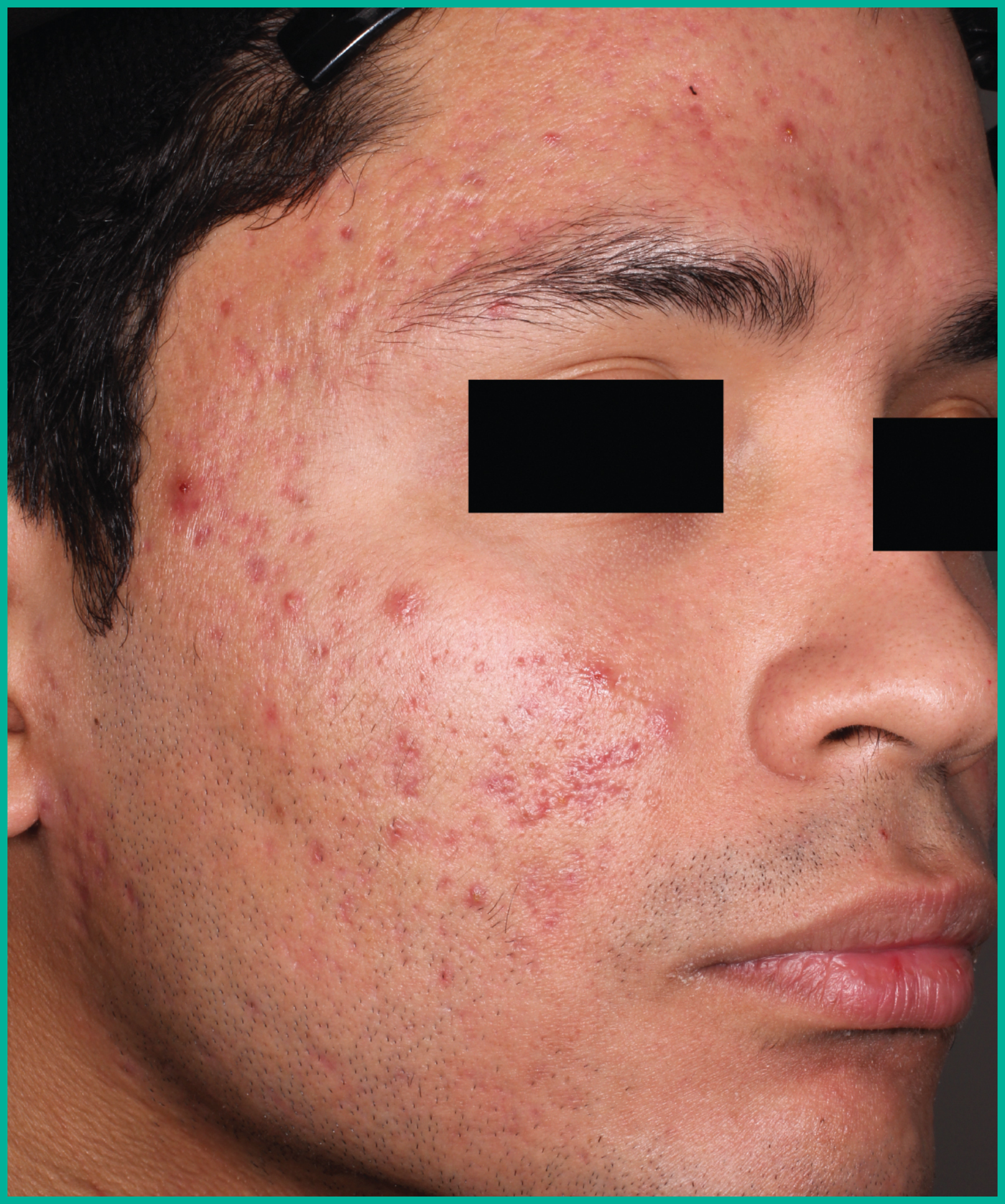
Treating acne with ARAZLO1
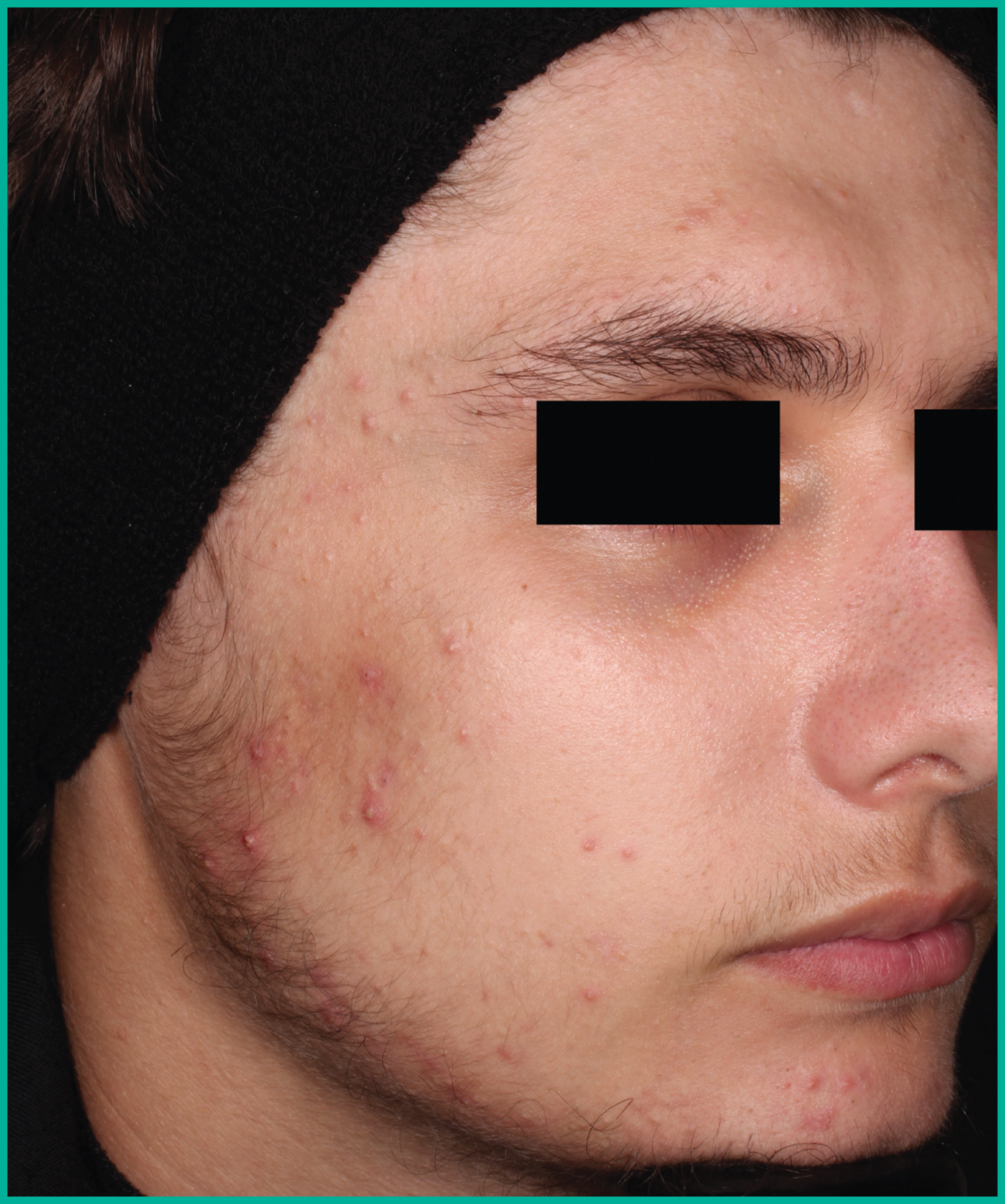
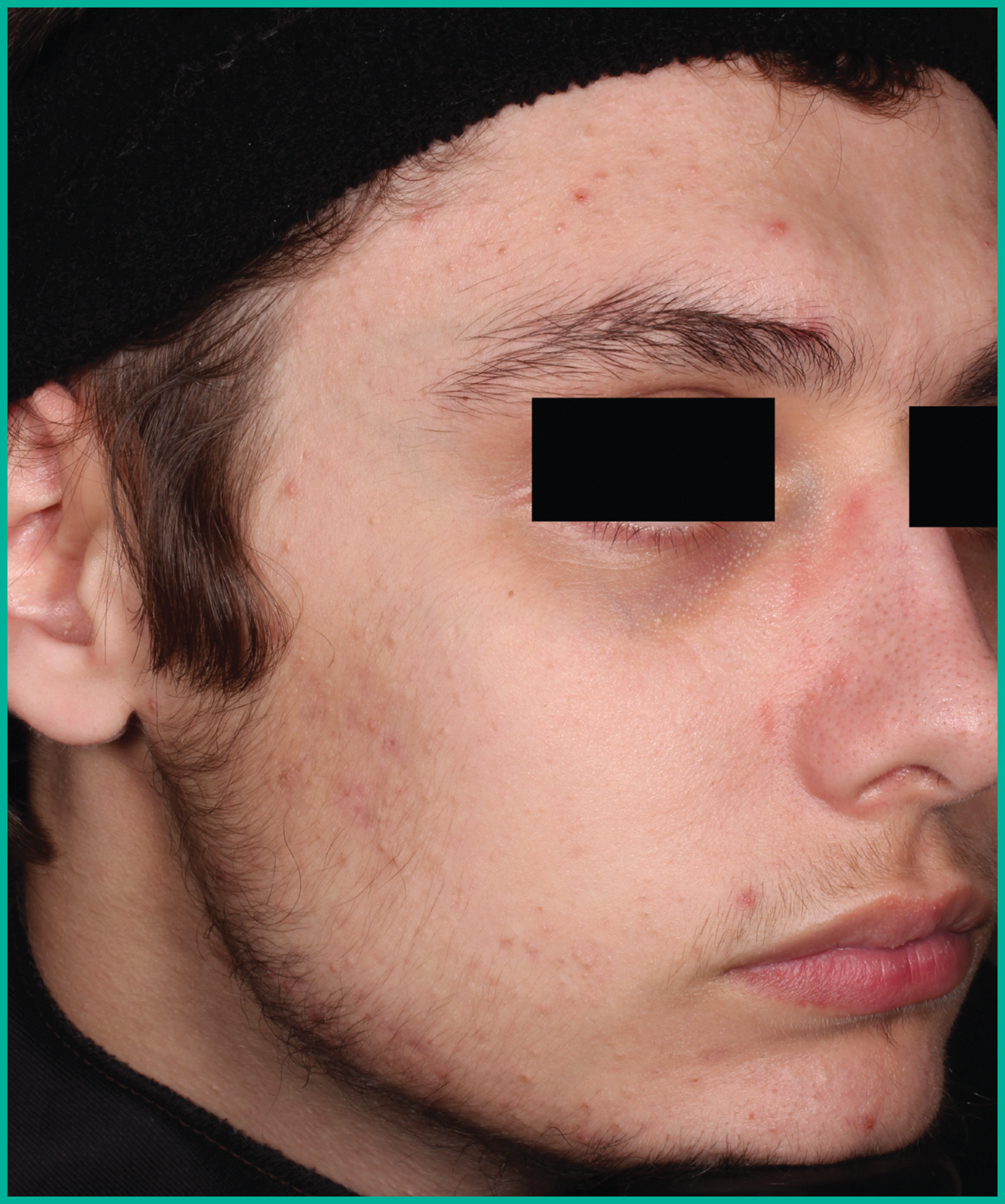
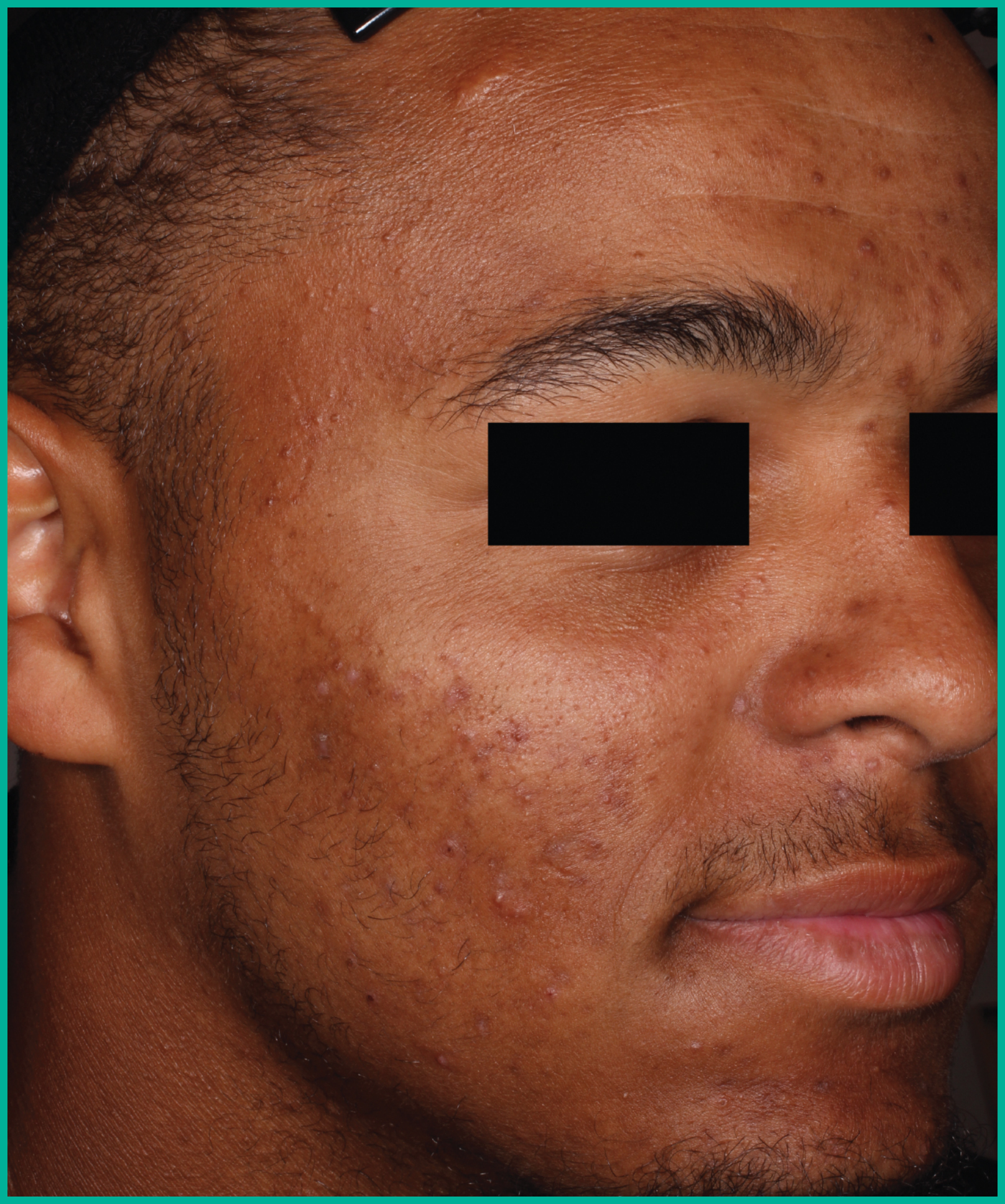
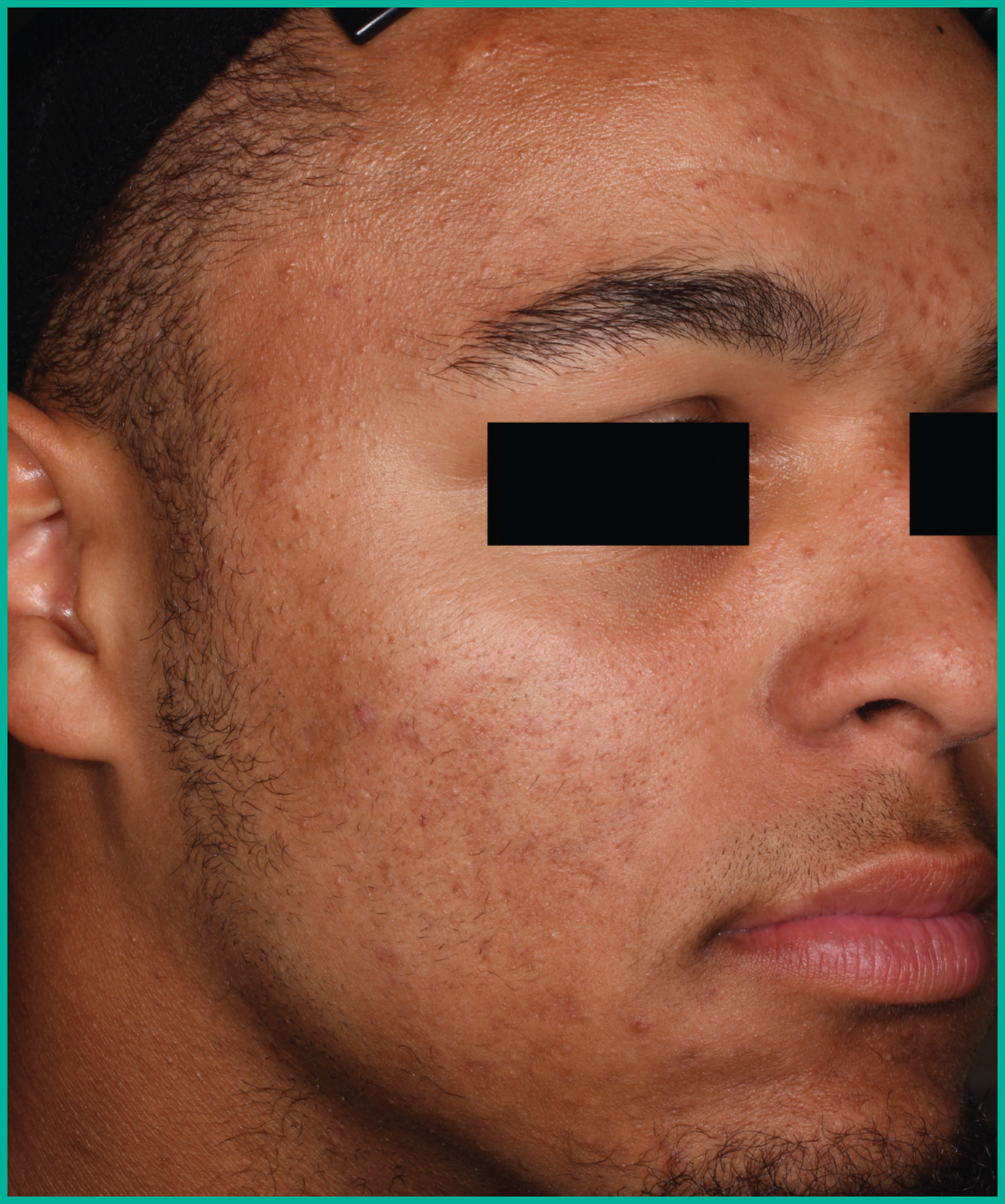
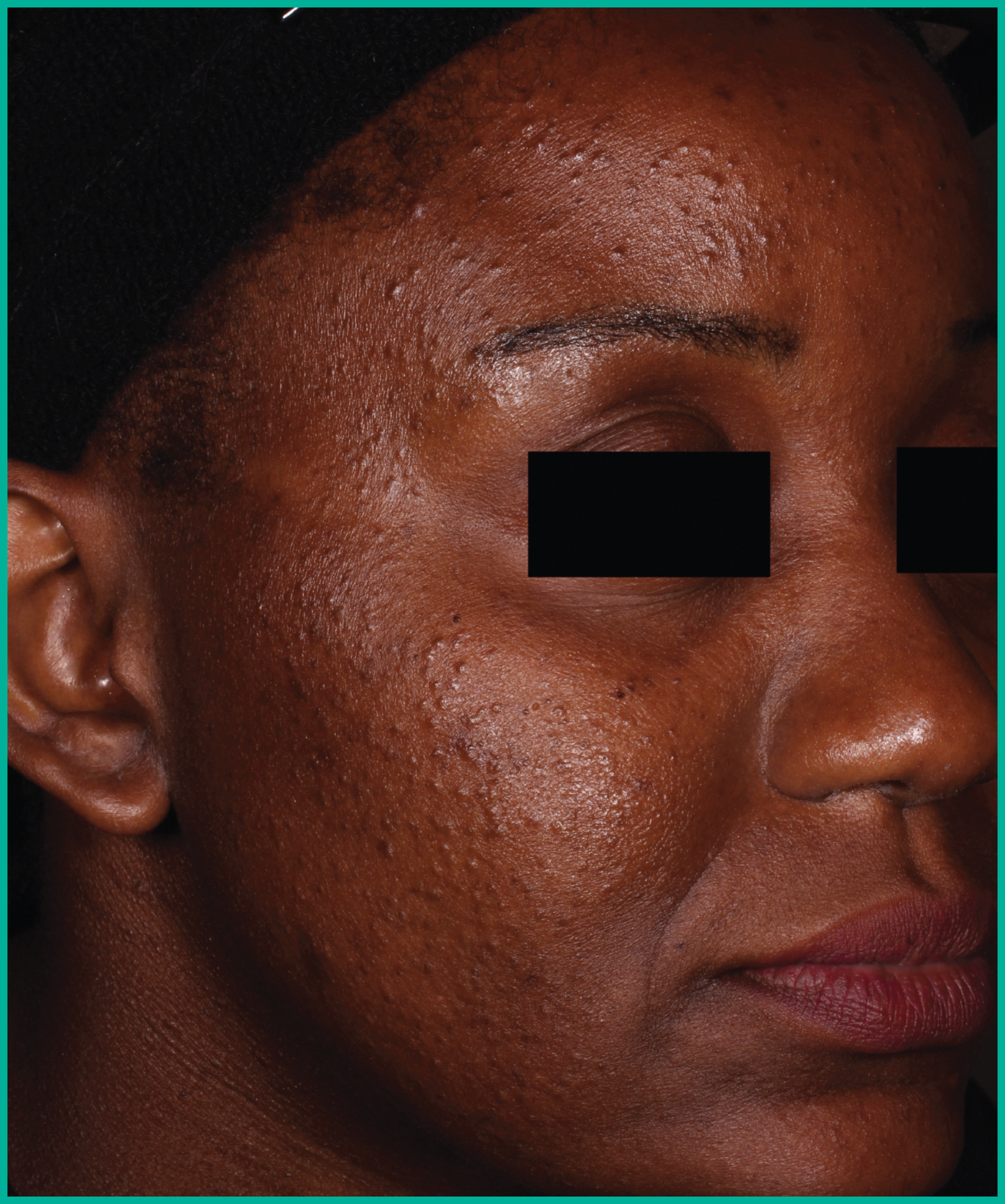
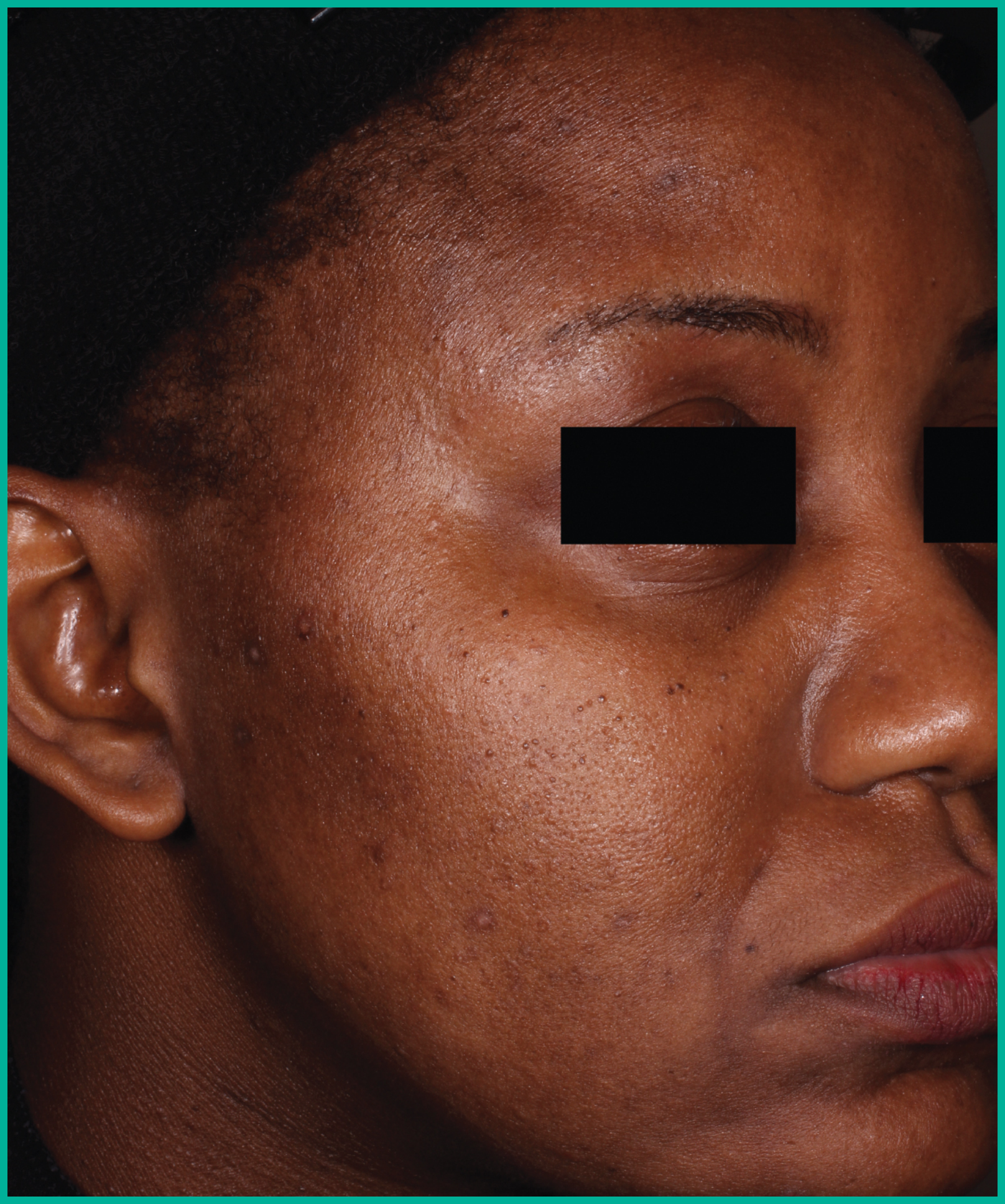
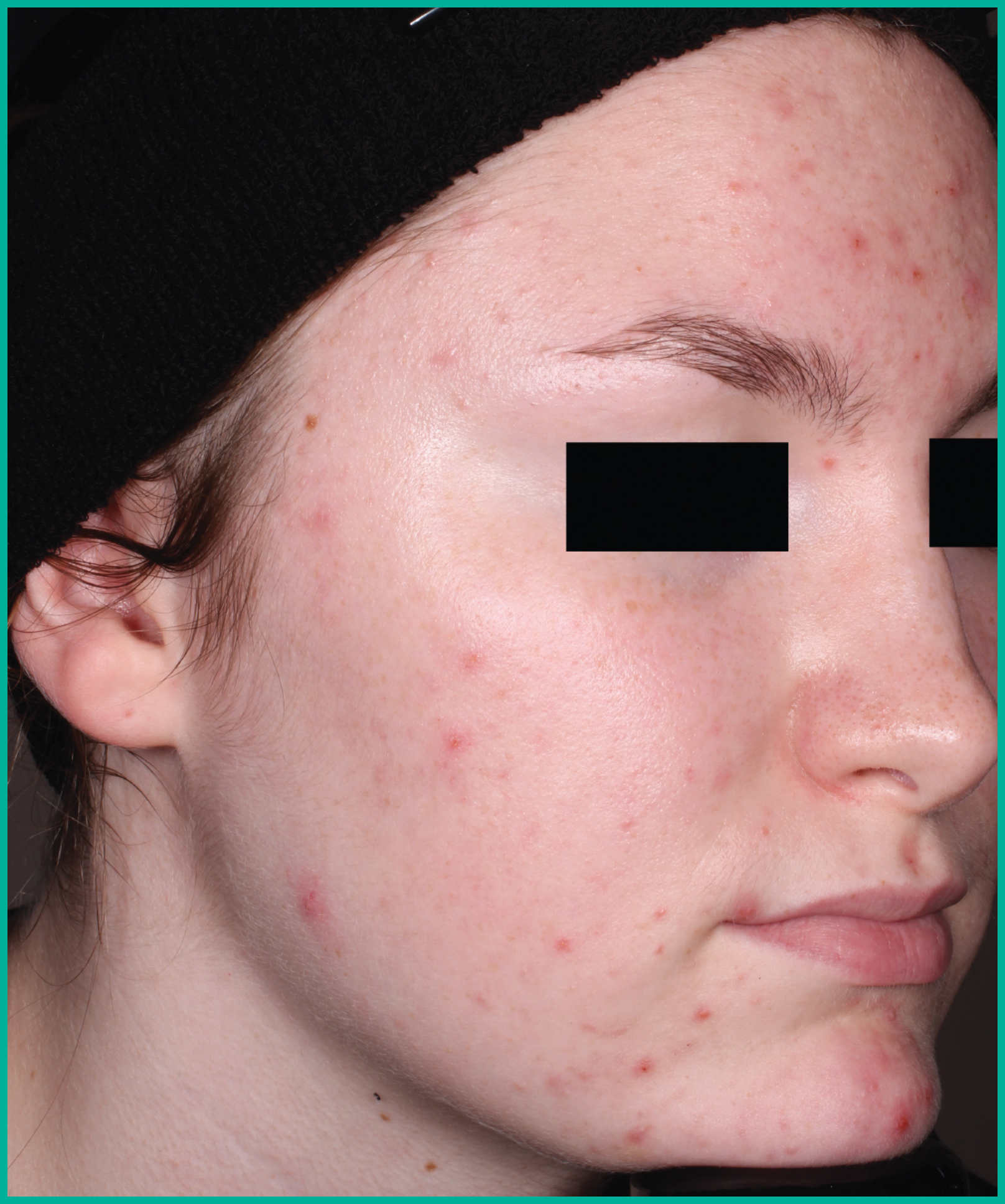
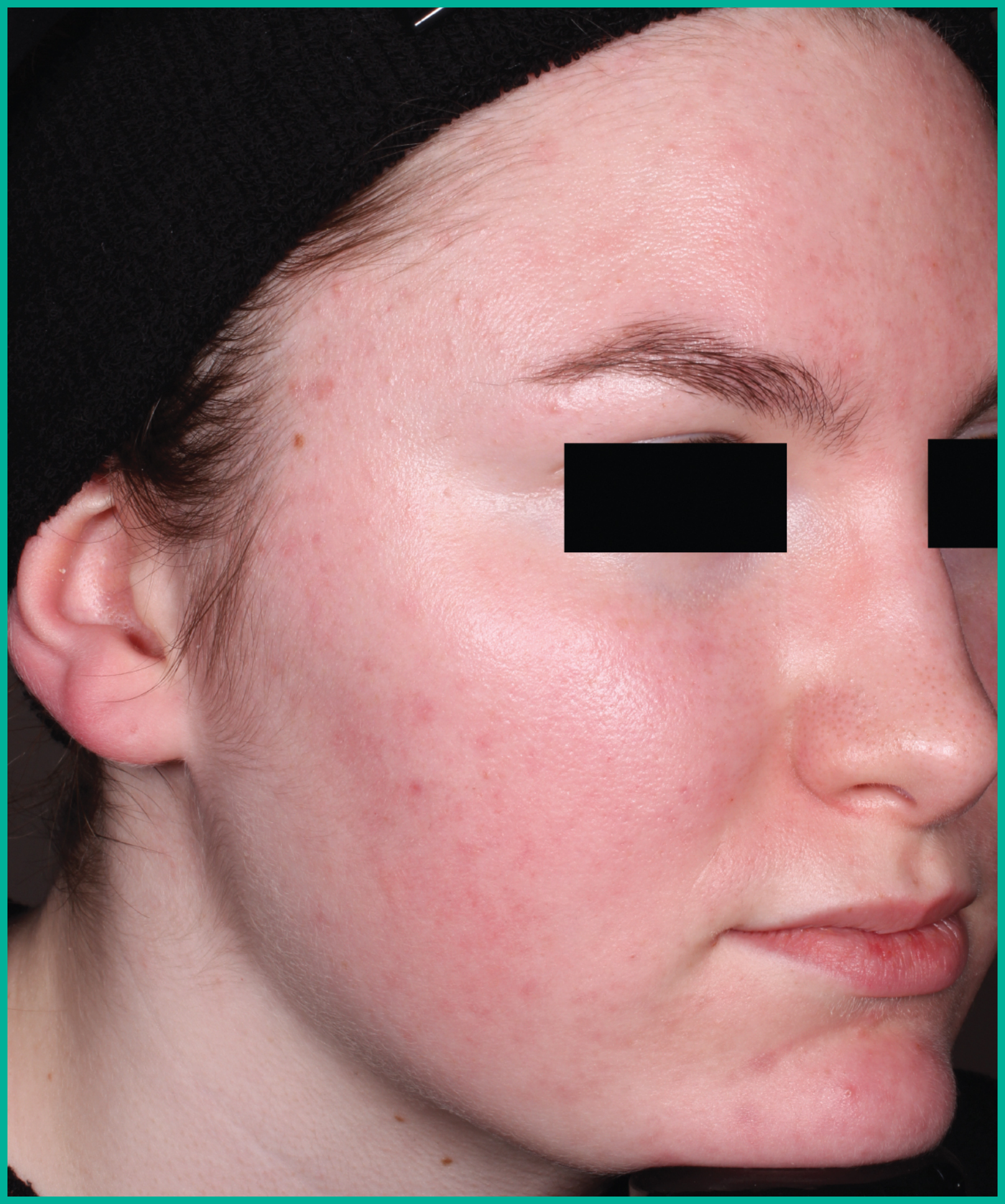
Patient results from pivotal clinical trials2
Drag the arrows to move between images
Two large vehicle-controlled studies involving more than 1600 patients combined*
ARAZLO demonstrated efficacy vs. vehicle at Week 12 for all co-primary endpoints in both studies1*
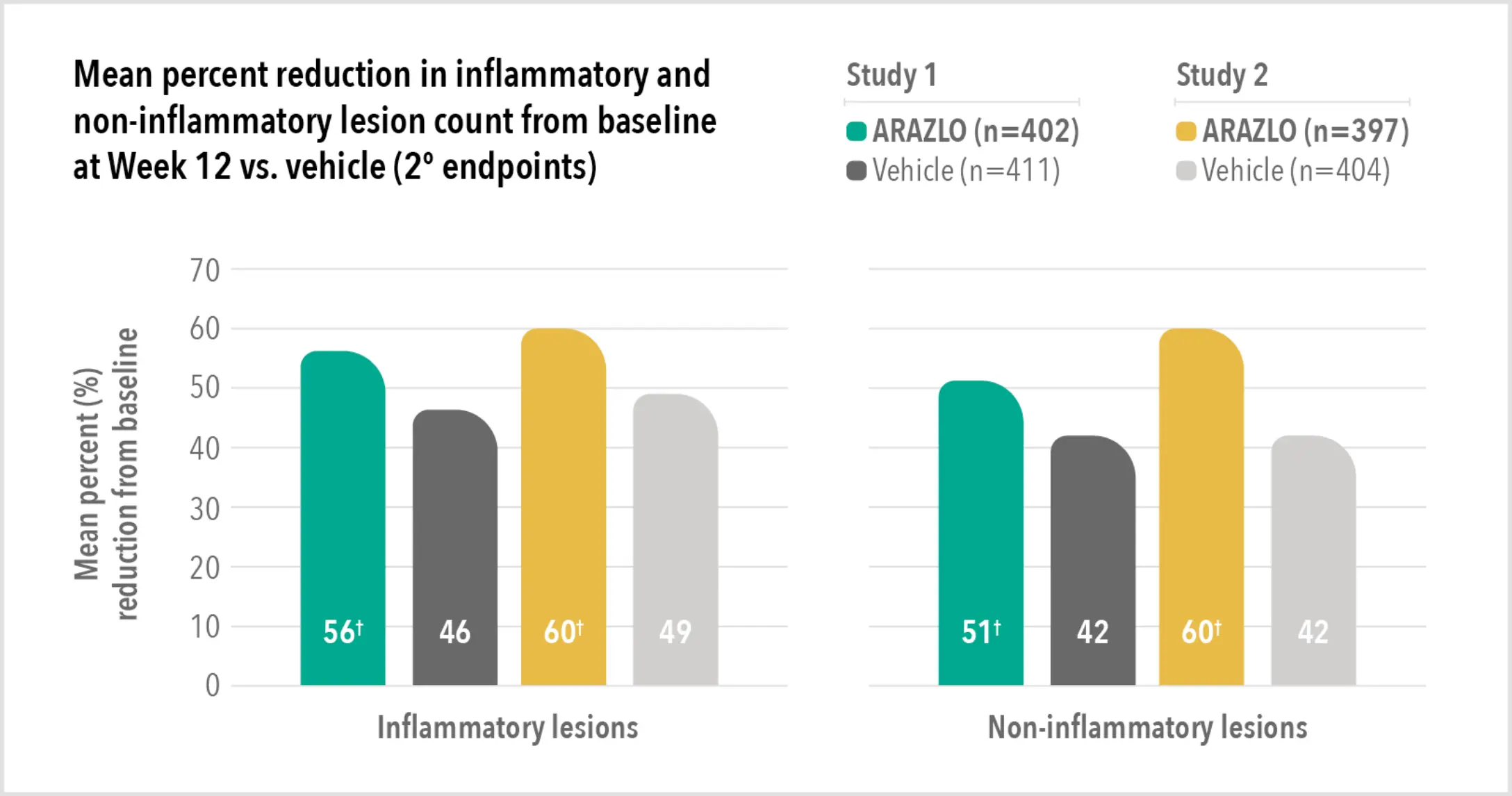
† p<0.001 vs. vehicle.
Adapted from Product Monograph.
Consult the Product Monograph for complete efficacy results.
Generally well tolerated1
In two pooled Phase III clinical trials:
- 97.2% (757/779) of patients remained on treatment‡
- Common topical AEs for ARAZLO (n=779) were: application site pain (5.3%), dryness (3.9%), exfoliation (2.1%), erythema (1.9%), and pruritus (1.3%)
Cutaneous safety and tolerability assessments§
- Overall, the incidence and mean scores of erythema, scaling, burning, stinging and itching were higher for ARAZLO vs. vehicle at any post-baseline visit
- Symptoms were generally mild to moderate, showing transient increases in severity that peaked around Week 2 and improved over the course of the study
At Week 12, most patients showed small changes from baseline in nearly all assessed cutaneous safety parameters
Consult the Product Monograph for complete information on incidence of cutaneous safety and tolerability parameters.
TEAE=treatment-emergent adverse event.
* Two identical, prospective, randomized, multicentre, double-blind, parallel-group, vehicle-controlled, Phase 3 clinical trials compared ARAZLO lotion (n=402 and n=397 in studies 1 and 2, respectively) with the vehicle lotion (n=411, n=404), in patients aged 9 years and older with moderate to severe acne. Secondary endpoints included percentage change in non-inflammatory and inflammatory lesion counts at Weeks 12, 8 and 4, and proportion of subjects with at least a 2-grade reduction from baseline in Evaluator’s Global Severity Score (EGSS) at Week 12.
‡ 2.8% (22/779) discontinued ARAZLO because of TEAEs.
§ Cutaneous safety and tolerability parameters (scaling, erythema, hypopigmentation, hyperpigmentation, itching, burning, and stinging) were evaluated at each study visit. Severity of the parameters was graded on a 4-point scale, where 0=none and 3=severe.

Once-daily application in 3 simple steps1
Here are some application tips to help counsel your patients when you prescribe ARAZLO.
If needed, patients may use a moisturizer after applying ARAZLO lotion – make sure patients allow skin to dry after applying ARAZLO lotion
Avoid the eyes, mouth, paranasal creases, and mucous membranes. If ARAZLO gets in or near eyes, rinse thoroughly with water. If ARAZLO is applied excessively, neither more rapid nor better results will be obtained and marked redness, peeling, or discomfort may occur. In this event, discontinue use and wait until the skin has recovered.
ARAZLO does not contain any fragrance, colourant or alcohol
If concomitant use of ARAZLO with oxidizing agents (e.g., benzoyl peroxide) is required, apply one in the morning and the other in the evening to separate application times
If needed, patients may use a moisturizer after applying ARAZLO lotion – remind them to allow sufficient drying time between applications
Remind patients that weather extremes, such as wind or cold, may be more irritating when using ARAZLO
Avoid concomitant use of medications and cosmetics that have a strong drying effect; postpone treatment with ARAZLO until drying effects subside
Exposure to sunlight, including sunlamps, should be avoided – instruct patients to use sunscreen with at least SPF 15 and wear protective clothing

Consider reducing frequency of ARAZLO application or discontinuing in cases of application site pain, dryness, exfoliation, erythema, pruritus; avoid application to eczematous or sunburned skin

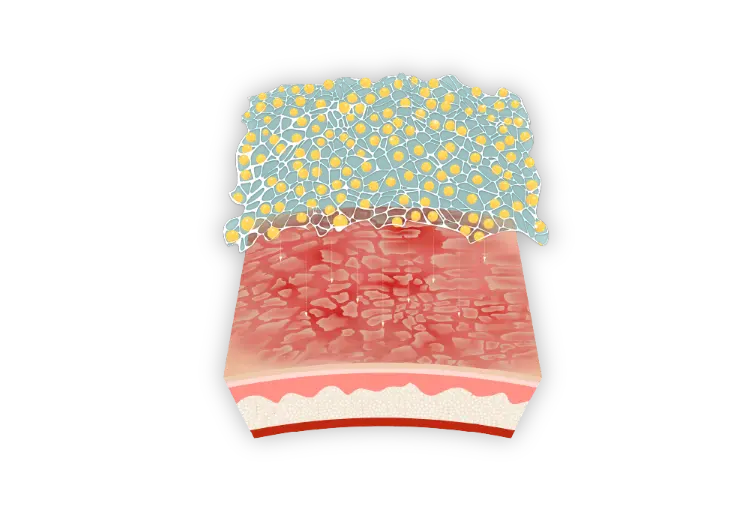
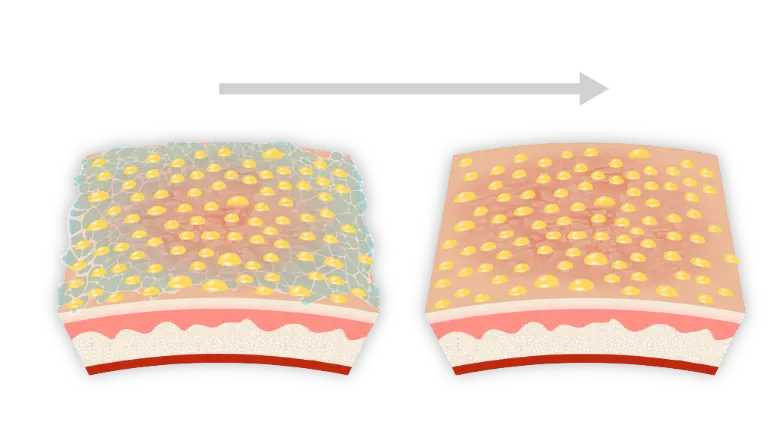
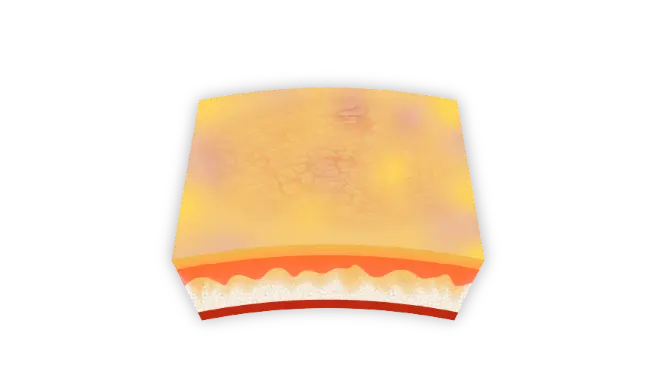
An in-depth look at PRISMATREXTM technology1
A novel vehicle formulation, developed by Bausch Health, used as the base for ARAZLO lotion
PRISMATREX is a three-dimensional mesh which allows for distribution of active ingredients throughout its matrix.1,3,4*
PRISMATREX has a target pH (5.0–6.0) that is consistent with the pH level tolerated by the skin without inducing irritation.1*
Hydrating/moisturizing ingredients† may alleviate skin dryness.1*
* Clinical significance unknown. † Diethyl sebacate, light mineral oil, and sorbitol solution.
ARAZLO is covered by all provincial formularies5–15
Hover over your province for listing details
Also covered under NIHB as an open listing
| Jurisdiction | Type of listing |
|---|---|
| British Columbia | Special authorization |
| Alberta | General benefit listing |
| Saskatchewan | Full benefit listing |
| Manitoba | Full benefit listing |
| Ontario | Limited use (LU code 636) For the treatment of acne vulgaris LU authorization period: 1 year |
| Quebec | Exceptional medication For treatment of acne (Code DE20) |
| Newfoundland and Labrador | Special authorization |
| Nova Scotia | Regular benefit for beneficiaries 30 years and under For the treatment of acne vulgaris for beneficiaries over the age of 30 |
| New Brunswick | Regular |
| Prince Edward Island | Open |
| NIHB | Open |
Product ordering information
ARAZLO
Tazarotene lotion 0.045% w/w
Format: 45 g
DIN: 02517868
Product code: 060752001762
UPC: 060752001762
- Patients 10 to <12 years of age should limit application of ARAZLO to the face
- Safety and efficacy of ARAZLO have not been established in patients >65 years of age
- People who are pregnant or may become pregnant
- Presence of seborrheic dermatitis
- For external topical use only
- Application site pain, dryness, exfoliation, erythema, and pruritus
- Use of topical tazarotene may produce contact dermatitis
- Avoid concomitant use of medications and cosmetics that have a strong drying effect
- Avoid application to eczematous or sunburned skin
- Photosensitivity
- Caution with coadministration of drugs known to be photosensitizers
- Use adequate birth-control measures in women of childbearing potential
- Breastfeeding
Consult the Product Monograph for important information on adverse reactions, drug interactions and dosing not discussed in this piece.
References: 1. ARAZLO Product Monograph. Bausch Health. 2. Data on file. Bausch Health. 3. Tanghetti EA, et al. J Dermatol Treat 2019; DOI: 10.1080/09546634.2019.1668907. 4. Metelitsa A, et al. Dermatology 2023;28(5):5–11. 5. Ontario Drug Benefit list. Arazlo. Available at: https://www.formulary.health.gov.on.ca/formulary/detail.xhtml?drugId=02517868. Accessed December 6, 2023. 6. Saskatchewan Formulary Bulletin #222. Available at: https://formulary.drugplan.ehealthsask.ca/Bulletins/Bulletin-0222-Nov-2022.pdf. Accessed December 6, 2023. 7. Régie de l’assurance maladie du Québec. List of Medications. Available at https://www.ramq.gouv.qc.ca/sites/default/files/documents/non_indexes/liste_med_2023-11-08_en.pdf. Accessed December 6, 2023. 8. Alberta Health. Interactive Drug Benefit List. Available at https://idbl.ab.bluecross.ca/idbl/drugDetails?_cid=39f1a0f1-0258-4f1a-86c9-eb8410f099e2&id=0000099458&intchg_grp_nbr=1&detailId=7749973. Accessed December 6, 2023. 9. Non-Insured Health Benefits. Drug Benefit List. Available at: https://nihb-ssna.express-scripts.ca/en/0205140506092019/16/160407. Accessed December 6, 2023. 10. Manitoba Drug Benefits and Manitoba Drug Interchangeability Formulary Amendments. Available at: https://web22.gov.mb.ca/eFormulary/searchResults.aspx?query=arazlo&type=basic. Accessed December 6, 2023. 11. Nova Scotia Formulary. Available at: https://novascotia.ca/dhw/pharmacare/documents/formulary.pdf. Accessed December 6, 2023. 12. Health PEI. PEI Pharmacare Formulary. Available at: https://www.princeedwardisland.ca/sites/default/files/publications/pei_pharmacare_formulary.pdf. Accessed December 6, 2023. 13. New Brunswick Drug Plans Formulary November 2023. Available at: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/NBDrugPlan/NewBrunswickDrugPlansFormulary.pdf. Accessed December 6, 2023. 14. NLPDP Drug Product Database. Newfoundland Labrador Health and Community Services. Available at: https://www.health.gov.nl.ca/health/prescription/class_search_nl.asp?din=02517868&GReturn=GenericName&Subtitle1=Generic%20or%20Brand%20Name%20:%20%20%20&Subtitle2=arazlo. Accessed December 15, 2023. 15. BC PharmaCare Limited Drug Coverage Program. BC PharmaCare. Available at: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/pharmacare/programs/limited-coverage-drug-program/limited-coverage-drugs-page-tazarotene-topical. Accessed April 12, 2024.